Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hanawa, Satoshi; Hata, Kuniki; Chimi, Yasuhiro; Nishiyama, Yutaka
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2013 (USB Flash Drive), 7 Pages, 2013/10
Water chemistry experiments will be carried out by using an in-pile loop newly installed in the JMTR. Concentrations of chemical species of O , H
, H and H
and H O
O are measured at the inlet and the outlet of the irradiation field. Electrochemical corrosion potential (ECP) at the irradiation field is also monitored. These experimental data will be obtained under wide range of experimental conditions such as absorption dose rate, H
are measured at the inlet and the outlet of the irradiation field. Electrochemical corrosion potential (ECP) at the irradiation field is also monitored. These experimental data will be obtained under wide range of experimental conditions such as absorption dose rate, H or O
or O concentration in the feeding water and water temperature. As a result of preliminary calculations, it became clear that the in-pile loop in the JMTR is capable for water chemistry experiment. Although the operation of the JMTR is being delayed because of the Tohoku district off the Pacific Ocean earthquake, construction of the loops and installation of the instrumentation for the loops have been carried out almost on schedule. The experiments will be started after JMTR restart.
concentration in the feeding water and water temperature. As a result of preliminary calculations, it became clear that the in-pile loop in the JMTR is capable for water chemistry experiment. Although the operation of the JMTR is being delayed because of the Tohoku district off the Pacific Ocean earthquake, construction of the loops and installation of the instrumentation for the loops have been carried out almost on schedule. The experiments will be started after JMTR restart.
 O
O
Sato, Tomonori; Kato, Chiaki; Ogi, Hirokazu*; Yamamoto, Masahiro; Ueno, Fumiyoshi
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2013 (USB Flash Drive), 6 Pages, 2013/10
The hydrogen peroxide (H O
O ) generated by the water radiolysis in the reactor coolant under irradiated condition plays an important role for the stress corrosion cracking (SCC) in the boiling water reactors (BWRs). In this study, the corrosive condition in high temperature water containing H
) generated by the water radiolysis in the reactor coolant under irradiated condition plays an important role for the stress corrosion cracking (SCC) in the boiling water reactors (BWRs). In this study, the corrosive condition in high temperature water containing H O
O was observed directly utilizing electrochemical impedance spectroscopy (EIS) in high temperature pure water. And the diffusion coefficient and thermal decomposition rate of H
was observed directly utilizing electrochemical impedance spectroscopy (EIS) in high temperature pure water. And the diffusion coefficient and thermal decomposition rate of H O
O at the 288
at the 288  C were estimated based on the obtained impedance responses. The reciprocal of the polarization resistance has linear relationship to the H
C were estimated based on the obtained impedance responses. The reciprocal of the polarization resistance has linear relationship to the H O
O concentration. The estimated diffusion coefficient of H
concentration. The estimated diffusion coefficient of H O
O (D
(D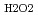 ) was 1.5
) was 1.5 10
10 cm
cm /s when the thickness of the diffusion layer was assumed to be 0.05 cm. The thermal decomposition rate of H
/s when the thickness of the diffusion layer was assumed to be 0.05 cm. The thermal decomposition rate of H O
O in 288
in 288 C was 0.042 s
C was 0.042 s .
.
 -ray irradiation experiment of aqueous solutions containing seawater components
-ray irradiation experiment of aqueous solutions containing seawater componentsHata, Kuniki; Hanawa, Satoshi; Kasahara, Shigeki; Motooka, Takafumi; Tsukada, Takashi; Muroya, Yusa*; Katsumura, Yosuke*
no journal, ,
Hydrogen peroxide production of  -ray irradiated artificial seawater and solutions containing each seawater component were measured. The concentration of hydrogen peroxide in each solution showed a similar trend to the results of radiolysis calculation. Hydrogen peroxide production of artificial seawater was quite similar to that of an aqueous solution, AQ1, which contains 2.9% sodium chloride, 82ppm sodium bromide and 193ppm sodium bicarbonate. Radiolysis calculation on AQ1 has shown that notable hydrogen production, which is also reported by some studies on seawater radiolysis. These results indicate that radiolysis of AQ1 is substituted for seawater radiolysis. Radiolysis calculation was also carried out to identify the seawater components which control the hydrogen peroxide production. It was found that the hydrogen peroxide production was determined by chemical reactions involving bromide ion and G values depending on the concentration of chloride ion.
-ray irradiated artificial seawater and solutions containing each seawater component were measured. The concentration of hydrogen peroxide in each solution showed a similar trend to the results of radiolysis calculation. Hydrogen peroxide production of artificial seawater was quite similar to that of an aqueous solution, AQ1, which contains 2.9% sodium chloride, 82ppm sodium bromide and 193ppm sodium bicarbonate. Radiolysis calculation on AQ1 has shown that notable hydrogen production, which is also reported by some studies on seawater radiolysis. These results indicate that radiolysis of AQ1 is substituted for seawater radiolysis. Radiolysis calculation was also carried out to identify the seawater components which control the hydrogen peroxide production. It was found that the hydrogen peroxide production was determined by chemical reactions involving bromide ion and G values depending on the concentration of chloride ion.